Abstract

Background Liposomal daunorubicin and cytarabine (CPX-351) improves survival compared to 3+7 chemotherapy in patients aged 60 years with secondary AML defined by clinical or cytogenetic criteria (Lancet JE, JCO 2018). It is increasingly recognised that secondary AML may be better defined by mutational profile than clinical history (Döhner, Blood 2022; Khoury, Leukemia 2022), however patients with molecularly defined secondary AML were not specifically included in previous studies. Moreover, no randomised data for CPX-351 in patients aged <60y were available prior to this study.
Previous UK NCRI trials established the FLAG-Ida regimen as a preferred regimen in patients aged <60y with high-risk and secondary AML (Burnett AK, Leukemia 2018). The UK NCRI AML19 trial randomised patients with AML or high grade MDS between induction therapy with CPX-351 and FLAG-Ida. Initial results reporting similar event-free and overall survival (OS) have been previously presented (Russell NH, HemaSphere 2022). Here we present an exploratory analysis of outcomes stratified by genomic, cytogenetic and clinical definitions of secondary AML.
Methods AML19 (ISRCTN78449203) enrolled patients with previously untreated AML, MDS-EB2, or MDS-EB1 with IPSS score >3.5, predominantly aged <60 years. Patients known to have an adverse karyotype at diagnosis according to MRC criteria were randomised between CPX-351 and FLAG-Ida. Treatment consisted of up to 4 courses of CPX-351 or 2 courses of FLAG-Ida followed by MACE/MidAC consolidation with allogeneic transplant recommended after 2 cycles if feasible. Of note, patients could also enter the FLAG-IDA vs CPX randomisation if they became high risk at other defined points after induction, the results of which will be reported separately.
Cytogenetic testing was performed in local laboratories with results reviewed and coded centrally. Complex karyotype was defined as ≥4 unrelated abnormalities. Following the completion of the trial, banked diagnostic DNA was analysed for variants in 41 recurrently mutated myeloid genes, including the entire coding regions of all myelodysplasia-related genes according to 2022 WHO and ELN criteria (ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2) (Döhner, Blood 2022; Khoury, Leukemia 2022). Libraries were prepared using the Agilent SureSelect XT HS2 platform and sequenced on an Illumina NextSeq2000 to a depth of >1000x.
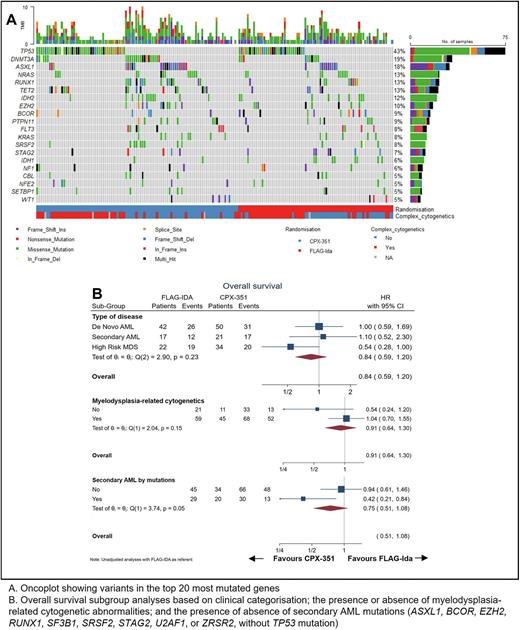
Results In total, 195 patients entered were randomised at trial entry, and NGS was performed on 173. Of the whole cohort, 49% were classified by clinical features as de novo AML, 20% as secondary AML and 31% as high-risk MDS. Myelodysplasia-related cytogenetic abnormalities were present in 70% of patients, with a complex karyotype in 51%. TP53 was the most commonly mutated gene in 43% of patients, followed by DNMT3A in 19% and ASXL1 in 18% (Figure A). A mutation in at least one myelodysplasia-related gene was present in 75 (43%) patients, of whom 60 (35%) were categorised as secondary AML by mutational status, which by ELN 2022 criteria requires the absence of TP53 variants.
As previously reported, OS did not differ between the two randomisation arms (Hazard Ratio [HR] for CPX-351 0.84, 95% confidence interval [95CI] 0.59 - 1.20). In patients with clinically defined secondary AML, there was no OS benefit for CPX-351 (HR 1.1, 95CI 0.53 - 2.30), while high-risk MDS had a trend to benefit (HR 0.54, 95CI 0.28 - 1.00) (Figure B). When secondary disease was defined by the presence of myelodysplasia-related cytogenetic abnormalities, CPX-351 did not provide benefit (HR 1.04, 95CI 0.77 - 1.55). However, in patients with mutationally defined secondary AML, there was an OS benefit from CPX-351 (HR 0.42, 95CI 0.21 - 0.84, p value for heterogeneity 0.05) (Figure B).
Patients with TP53 mutations had an adverse prognosis, with median OS of 7 months compared to 28 months in those with wild-type TP53. CPX-351 did not provide benefit compared to FLAG-Ida in this group (HR 0.92, 95CI 0.57 - 1.49).
Conclusion In this exploratory subgroup analysis, CPX-351 had a significant survival benefit over FLAG-Ida in young high-risk patients with secondary AML/MDS as defined by the presence of myelodysplasia-related gene mutations, but not by clinical criteria. We observed no significant difference in patients with TP53 mutations.
Acknowledgements This study received research support from Cancer Research UK and a research grant from Jazz Pharma
Disclosures
Dillon:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Research Funding; Astellas: Consultancy, Honoraria, Speakers Bureau; Avencell: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Knapper:Novartis: Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria; BMS: Consultancy; Astellas: Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau. Mehta:Jazz: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Astellas: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Russell:Pfizer: Honoraria, Research Funding, Speakers Bureau; Jazz Pharma: Research Funding; Servier: Honoraria; Astellas: Honoraria.
OffLabel Disclosure:
CPX-351 is FDA approved for the treatment of adults with newly diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC). This presentation will describe the use of CPX-351 in adults with AML with adverse karyotype and high-risk AML, which includes some patients who do not meet the FDA label
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal